![]()
 |
 |
|
![]()
LIVERMORE SCIENTISTS TEAM WITH RUSSIA
|
||||||||||||||||||||||||||||||||||||||||||||||||||
LIVERMORE, California —February 2, 2004 |
||
Scientists from the Glenn T. Seaborg Institute and the Chemical Biology and Nuclear Science Division at the Lawrence Livermore National Laboratory, in collaboration with researchers from the Joint Institute for Nuclear Research in Russia (JINR), have discovered the two newest super-heavy elements, Element 113 and Element 115. In experiments conducted at the JINR U400 cyclotron with the Dubna gas-filled separator between July 14 and August 10, 2003, the team of scientists observed atomic decay patterns, or chains, that confirm the existence of Element 115 and Element 113. In these decay chains, Element 113 is produced via the alpha decay of Element 115. The results have been accepted for publication in the February 1, 2004, issue of Physical Review C. “These elemental discoveries underscore both the value of federally-supported basic research and the benefit of unfettered international scientific collaboration,” Secretary of Energy Spencer Abraham said. The experiments produced four atoms each of Element 115 and Element 113 through the fusion reaction of Calcium-48 nuclei impinging on an Americium-243 target: 95Am243 + 20Ca48 → 115UUP288 + 30n1 95Am243 + 20Ca48 → 115UUP287 + 40n1 The team observed three similar decay chains consisting of five consecutive alpha decays that combined took less than 30 seconds and terminated in a spontaneous fission of an Element 105, Dubnium, isotope with a very long half-life (16 hours), making the discovery of particular interest to chemists. An interesting fourth decay chain also was observed that consisted of decays that were unlike the previous three chains. Joshua Patin, Livermore’s primary data analyst on the team, said the three similar decay patterns were a “positive identifier that something good had been seen because the long decay chains just don’t happen that often.” “This just opens up the horizon on the periodic table,” said Ken Moody, Livermore’s team leader. “It allows us to expand the fundamental principles of chemistry. From new chemistry comes new materials and new technology.” Scientists at Livermore and JINR independently verified the data. An efficient accelerator is needed to obtain an intense Calcium-48 beam. The results have only been achieved to date on the JINR’s U400 cyclotron. Associates at JINR’s ion-source group produced the intense Calcium beams while Livermore supplied the Americium target material. “Twenty years ago, no one would have ever thought that this was possible because the technology to produce such an element just wasn’t there,” Patin said. “But with the efficiency of the Russian cyclotron and the ability to run the experiments for long periods of time, we were able to achieve this tremendous accomplishment.” Members of the Livermore team include Patin, Moody, John Wild, Mark Stoyer, Nancy Stoyer, Dawn Shaughnessy, Jacqueline Kenneally and Ronald Lougheed. Livermore has had a long-standing heavy element group since the inception of the Laboratory in 1952. The group has been successful in the discovery of several new elements over the years because it has access to very unique materials to perform the experiments. In 1998 and 1999, the Laboratory announced the discovery of Elements 114 and 116, respectively. “This is quite a breakthrough for science,” said Chemistry and Materials Science Associate Director Tomas Diaz de la Rubia. “We’ve discovered two new elements that provide insight into the makeup of the universe. “For our scientists to find two more pieces of the puzzle is a testament to the strength and value of the science and technology at this Laboratory.” Scientists in Livermore’s Seaborg Institute, named after the renowned nuclear chemist, reinvent nuclear and bionuclear science to enable out-of-the-box solutions to national problems. The work is supported by the Russian Ministry of Atomic Energy and the U.S. Department of Energy as part of the Russian Federation/U.S. Joint Coordinating Committee for Research on Fundamental Properties of Matter. |
 A Calcium-48 ion is accelerated to a high velocity in a cyclotron and directed at an Americium-243 target. |
|
 One of the numerous Americium-243 target atoms with a nucleus of protons and neutrons surrounded by an electron cloud. |
||
 An accelerated Calcium-48 ion and an Americium-243 target atom just before they collide. |
||
 The moment of collision between an accelerated Calcium-48 ion and an Americium-243 target atom. |
||
 The residue of the collision creates the new Element 115 that begins decaying with the emission of alpha particles into Element 113. |
||
 The spontaneous fission decay eventually results in two separate atoms of previously known elements. |
||
![]()
Founded in 1952, Lawrence Livermore National Laboratory is a national security laboratory, with a mission to ensure national security and apply science and technology to the important issues of our time. Lawrence Livermore National Laboratory is managed by the University of California for the U.S. Department of Energy’s National Nuclear Security Administration.
References: |
http://www.llnl.gov/llnl/06news/NewsReleases/2004/NR-04-02-01.html: |
http://159.93.28.88/flnr/elm113p.html: |
|
http://www.webelements.com/webelements/elements/text/Uut/key.html: |
|
http://www.webelements.com/webelements/elements/text/Uup/key.html: |
![]()
Scientists from the Glenn T. Seaborg Institute and the Chemical Biology and Nuclear Science Division at the Lawrence Livermore National Laboratory, in collaboration with researchers from the Joint Institute for Nuclear Research in Russia (JINR), have discovered a new super-heavy element, Element 116. An isotope of Element 116, 116UUH292, was identified in the reaction of Curium, 96Cm248, with Calcium, 20Ca48. This isotope has a very short half-life and decomposes to a previously discovered isotope of Element 114, 114UUQ288.
On December 6, 2000, results were published concerning recent experiments at Dubna in Russia (involving workers from The Joint Institute for Nuclear Research, Dubna, Russian Federation; The Lawrence Livermore National Laboratory, California, USA; The Research Institute of Atomic Reactors, Dimitrovgrad, Russian Federation; and The State Enterprise Electrohimpribor, Lesnoy, Russian Federation) describing the creation of Element 116 isotope 116UUH292 resulting from the reaction of 96Cm248Cm with 20Ca48:
96Cm248 + 20Ca48 → 116UUH292 + 40n1
This isotope of Element 116, 116UUH292, decayed in 47 milliseconds to a previously identified isotope of Element 114, 114UUQ288:
116UUH292 → 114UUQ288 + 2He4
References: |
http://education.jlab.org/itselemental/ele116.html: |
http://159.93.28.88/flnr/elm116p.html: |
|
http://www.webelements.com/webelements/elements/text/Uuh/key.html: |
![]()
KNOXVILLE, Tennessee (June 6, 1999 11:14 a.m. EDT) - King Arthur had a holy grail; Witek Nazarewicz has his “magic nuclei.”
Nazarewicz is a theoretical physicist in search of elements with atoms that are stable yet jam-packed with protons and neutrons in their nuclei. Because of their potential atomic weight, they’re called “the super heavies.”
In his quest for them, Nazarewicz, of Oak Ridge National Laboratory and the University of Tennessee, has taken a major step forward. He may have helped identify a new element to be added to the periodic table: Number 114, for the record, called “UnUnQuadium” for now.
Holding degrees from Warsaw University of Technology, Nazarewicz is a native of Poland whose enthusiasm for his science is obvious. During an interview he pulled out a handful of charts and scribbled diagrams on paper to illustrate the principles behind the potential breakthrough.
“The data are not 100 percent conclusive, of course, but what we have found is evidence for the possibility of a new element. The evidence came through a set of very complicated calculations. A super computer sweats for weeks and then you get a number from Europe,” he said, smiling.
The team effort involved Nazarewicz and his ORNL colleagues, physicists at the Joint Institute for Nuclear Research in Dubna, Russia, and at California’s Lawrence Livermore National Laboratory.
Last November and December, the physicists at Dubna and Lawrence Livermore spent 40 days bombarding Plutonium targets with Calcium ions. Until then, Nazarewicz said, scientists had believed that process would result in a reaction that would cause fission to occur before an element could be created.
Of the vast number - 10 to the 18th power, to be exact - of collisions created by running the experiment, “one decay chain stood out as a candidate to be a new element, Number 114,” according to UT Physics Department officials.
Meanwhile, Nazarewicz and fellow researchers from Warsaw and Brussels, Belgium, had been working for years on mathematical models to determine the limits of atomic mass. Upon hearing of the possible element created by the experiment last fall, Nazarewicz and his team offered their models for comparison.
“What was found was remarkable agreement between our theory and their experiment,” he said. “I felt great about it. I feel, thanks to our work, there’s great confidence this is the stuff. It’s very gratifying.”
The milestone has been outlined in two papers - one from Dubna dealing with the experimental work, another from Nazarewicz and his colleagues on the theoretical side - submitted to the scientific journal, Physical Review Letters.
Nazarewicz said he expects the GSI group, “the German equivalent of our national laboratory system,” to be among the first researchers who’ll try to verify the work.
In scientific history, the “super heavies” mark a fourth period of radioactive element discovery, UT officials said.
The first period and beginning of this radioactive era brought the work of Marie and Pierre Curie. They launched the atomic age 100 years ago with their discovery that not all nuclei are stable - that some are radioactive. They also discovered Polonium - Number 84 - named for Poland, Marie Curie’s native country.
The Curies’ discoveries led physicists to wonder what the limits of charge and mass for a nucleus might be. Scientists found that by manipulating the numbers of protons and neutrons, they could synthesize elements in laboratories.
Which is what went on during the second period of radioactive element discovery, marked by the Manhattan Project, during which Plutonium - Number 94 - took its place on the Periodic Table. The third time period was essentially the Cold War competition waged between Russian laboratories at Dubna and American labs in Berkeley, California, for the discovery of new elements.
The current, or fourth, period has been dominated by work in Darmstadt, Germany, which Nazarewicz said has been the source of the six new elements found since 1981.
That year, Bohrium - Element 107 and named for Danish physicist Niels Bohr - became the first member of the “super heavy” class, Nazarewicz said.
“From that up to 112, all belong to the Germans. Then the Germans ran out of steam - even the Germans,” he said and laughed. “Actually, they had approached the limits of their technical capability.”
From element Number 112 to Number 114, a “dramatic jump” - from microseconds to 30 seconds - in the elements’ half-lives were found, Nazarewicz said.
“For some, the question of existence may mean years or ages, but for me, if something exists for a second, this is a long time,” he said. “That half-life of 30 seconds is fairly long.”
The nuclear reaction producing the Element 114 isotope 114UUQ289 containing 175 neutrons with a 30 second half-life is provided below:
94Pu244 + 20Ca48 → 114UUQ289 + 30n1
The nuclear reaction producing the Element 114 isotope 114UUQ288 containing 174 neutrons with a half-life of 2 seconds is provided below:
94Pu244 + 20Ca48 → 114UUQ288 + 40n1
And what are these elements? Do they occur in nature?
“We don’t know. That’s hard to say,” Nazarewicz said. “It may be that some cosmic explosions in space could be the source of the kinds of events necessary to produce them. I doubt they could be produced in massive amounts.”
“But as we say in physics, ‘Always expect the unexpected.’ For those of us who are proud of being explorers, this is certainly the case.”
The so-called magic nuclei Nazarewicz seeks are “the longest-lived super-heavy elements.” Creating them means creating a blueprint for mapping out the nuclear landscape’s uncharted territory, what he calls the “terra incognita” – “Or to see how far you can go in atomic mass – how heavy you can make the stuff.”
Element 112 remains the last confirmed “super heavy,” discovered in 1996. Like Elements 110 and 111 before it, Element 112 remains unnamed “because of politics,” Nazarewicz said. He said Element 113 might be a more difficult experiment to produce than Element 114 as the scientific world moves closer to their identification.
Naming disputes led the International Union for Pure and Applied Chemistry to develop a system based on Latin to issue temporary names based on individual numbers.
Clearly pleased at the accolades for working to identify “UnUnQuadrium,” Nazarewicz gets an even brighter twinkle in his eye as he talks of promise held by the future.
“Sometimes research doesn’t have to have an immediate payback. Pure research by pure scientists has changed the world before,” he said. “Look at the LASER. That was a discovery by pure scientists. So was the transistor, and now those are everywhere.”
Reference: |
http://www.webelements.com/webelements/elements/text/Uuq/key.html: |
![]()
DISCOVERY OF two new “super-heavy” elements has been announced by scientists at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory. Element 118 and its immediate decay product, Element 116, were discovered at Berkeley Lab’s 88-Inch Cyclotron by bombarding targets of Lead with an intense beam of high-energy Krypton ions, according to a press release.
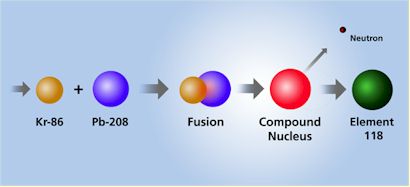
| NOTE: | In 1999, California and Oregon State University researchers bombarded a lead target with a beam of Krypton ions. They reported detecting three atoms of Element 118, which then was the heaviest element detected. They decayed almost instantly into Element 116. This article, “Two New Super-Heavy Elements Discovered,” is the very story of this reported breakthrough. But two years ago, the claims were retracted after a scientist at the Lawrence Berkeley National Laboratory was found to have fabricated data. Physicist Victor Ninov was the only member of the lab’s 16-member team to be dismissed in the incident, and he is appealing the decision. Other researchers later created Element 116. |
“Although both new elements almost instantly decay into other elements, the sequence of decay events is consistent with theories that have long predicted an ‘island of stability’ for nuclei with approximately 114 protons and 184 neutrons. We jumped over a sea of instability onto an island of stability that theories have been predicting since the 1970s,” said nuclear physicist Victor Ninov who was first author of a paper that has been submitted to Physical Review Letters.
Said Ken Gregorich, a nuclear chemist who led the discovery team, “We were able to produce these super-heavies using a reaction that, until a few months ago, we had not considered using. However, theoretician Robert Smolanczuk (a visiting Fulbright scholar from the Soltan Institute for Nuclear Studies in Poland) calculated that this reaction should have particularly favorable production rates.
Our unexpected success in producing these superheavy elements opens up a whole world of possibilities using similar reactions: New elements and isotopes, tests of nuclear stability and mass models, and a new understanding of nuclear reactions for the production of heavy elements.”
Gregorich and Ninov are members of Berkeley Lab’s Nuclear Science Division (NSD). Walter Loveland, on sabbatical from Oregon State University, also made major contributions to this work.
The isotope of Element 118 with mass number 293 identified at Berkeley Lab contains 118 protons and 175 neutrons in its nucleus. By comparison, the heaviest element found in Nature in sizeable quantities is Uranium which, in its most common form, contains 92 protons and 146 neutrons.
Transuranic elements in the periodic table can only be synthesized in nuclear reactors or particle accelerators. Though often short-lived, these artificial elements provide scientists with valuable insights into the structure of atomic nuclei and offer opportunities to study the chemical properties of the heaviest elements beyond Uranium.
Within less than a millisecond after its creation, the Element 118 nucleus decays by emitting an alpha particle, leaving behind an isotope of Element 116 with mass number 289, containing 116 protons and 173 neutrons.
This daughter, Element 116, is also radioactive, alpha-decaying to an isotope of Element 114. The chain of successive alpha decays continues until at least Element 106.
“In these experiments, observation of a chain of six high-energy alpha decays within about one second unambiguously signaled the production and decay of Element 118,” says Gregorich. “During 11 days of experiments, three such alpha-decay chains were observed indicating production of three atoms of Element 118.
The decay energies and lifetimes measured for these new isotopes of Elements 118, 116, 114, 112, 110, 108, and 106 provide strong support for the existence of the predicted island of stability.”
Referring to these results, discovery-team member Hoffman said, “After a 30-year search, this discovery is extremely gratifying. I only wish Glenn Seaborg had been alive to see these results.” Seaborg, the recently deceased Nobel laureate chemist and co-discoverer of Plutonium and nine other transuranic elements, was one of the earliest and most outspoken advocates of experiments to reach the predicted island of stability.
Elements 118 and 116 were discovered by accelerating a beam of Krypton-86 ions to an energy level of 449 million electron volts and directing the beam into targets of Lead-208. This yielded heavy compound nuclei at low excitation energies.
During the last several years, low excitation energy reactions failed to take scientists beyond Element 112, and it was assumed that production rates for heavier elements were too small to extend the periodic table further using this approach. However, the recent calculations of Smolanczuk indicating increased production rates for the Kr-86 + Pb-208 reaction prompted the experimental search for Element 118 at Berkeley Lab.
The key to the success of this experiment was the newly constructed Berkeley Gas-filled Separator (BGS). Said Gregorich, “The innovative BGS design has resulted in a separator with unsurpassed efficiency and background suppression which allows us to investigate nuclear reactions with production rates smaller than one atom per week.
For these experiments, the strong magnetic fields in the BGS focused the Element 118 ions and separated them from all of the interfering reaction products which were produced in much larger quantities.”
Another important factor for the experiment’s success was the unique ability of the 88-Inch Cyclotron to accelerate neutron-rich isotopes such as Krypton-86 to high-energy and high-intensity beams with an average current of approximately 2 trillion ions per second.
“The 88-inch Cyclotron is the only accelerator in the United States at this time that can provide Krypton beams at the intensities that this experiment demanded,” said Claude Lyneis, the NSD physicist who heads the accelerator facility for Berkeley Lab.
In operation since 1961, the 88-inch Cyclotron has been upgraded with the addition of a high-performance ion sources and can now accelerate beams of ions as light as Hydrogen or as heavy as Uranium. The 88-inch Cyclotron is a national user facility serving researchers from around the world for basic and applied studies.
Said I-Yang Lee, scientific director at the 88-inch Cyclotron, “From the discovery of these two new superheavy elements, it is now clear that the island of stability can be reached.
Additionally, similar reactions can be used to produce other elements and isotopes, providing a rich new region for the study of nuclear and even chemical properties.”
| References: | http://www.aip.org/physnews/graphics/html/element118.html: “Two New Super-Heavy Elements Discovered” |
|
|
||
| http://www.lbl.gov/Science-Articles/Research-Review/Magazine/1999/departments/breaking_news.shtml: “Discovery of New Elements Makes Front Page News” |
||
![]()
The physicist Victor Ninov, born in Bulgaria, received his PhD in 1992 for his studies at the Heavy Ion Research Center (GSI) in Darmstadt (Germany). He continued his research there till 1997, then he went to Berkeley (USA).
At Lawrence Berkeley National Laboratory (LBNL) in California, he was a member of the group which announced the creation of Elements 116 and 118 in 1999. This was seen as a major step forward to the island of stability which was conjured around Element 120.
Theoretical physicists thought that Element 118 could hardly be manufactured. Robert Smolanczuk suggested that a Krypton beam hitting a lead target might provide a higher likelyhood. And that was what they did in Berkeley. During a 10 days run, they claimed to have discovered three alpha decay chains of Element 118.
But other research groups around the world failed to duplicate these results, also a new experiment at Berkeley failed. Hence, the LBNL retracted the publication of the discovery in July 2001. But that retraction was not published by the scientific journal, because one of the former authors was missing: Victor Ninov.
Even worse, the LBNL could not find original records of the data which contained the original proof for the Element 118 and its decay via Element 116.
They looked more closely at the case, and then they proceeded to a formal investigation of Ninov’s conduct under the provisions of LBNL’s stated policy on integrity in research.
Then they found out that Ninov had fabricated the decisive data for the proof. The Elements 116 and 118 rested on a web of false data.
During a re-analysis of the data for the Elements 111 and 112 at the GSI in Darmstadt, little falsifications committed by Ninov since 1994 came to light. But these were small falsifications only which did not alter the meaning of the experiments and the conclusions drawn therefrom.
Ninov was placed on paid leave in November 2001 and fired in May 2002. Ninov vehemently contradicts to the accusations against him.
| References: | http://home.t-online.de/home/Bernhard.Hiller/fraud-41.htm: “A Case of Fraud: Element 118 (Victor Ninov)”
|
|||
| http://www.lbl.gov/Science-Articles/Archive/118-retraction.html: “Results of Element 118 Experiment Retracted” |
||||
| http://pubs.acs.org/cen/topstory/8029/8029notw6.html: “SUPERHEAVY FUROR: Berkeley Lab Fires Physicist for Fraud Surrounding Elements 116 and 118” |
||||
![]()
HYPERLINKS:
Index and Direct Hyperlinks to the Other Web Pages on this Website:
![]()